Abstract
Background:
Current literature suggests that innovative model-based dose escalation schemes in phase 1 trials estimate the maximum tolerated dose of a drug more precisely and efficiently. Additional strategies to meet enrollment needs for phase I studies include multi-center trial design, broader inclusion of patients, and use of expansion cohorts.
There has been a significant growth in novel therapies in hematological malignancies over recent years. However, it is unclear whether newer phase I trial methodologies have been employed to assess these treatments.
Methods:
A systematic review of MEDLINE and EMBASE between 2005 and 2015 was performed to identify all phase I and phase I/II trials in adult patients with hematologic malignancies. Trials involving autologous and allogeneic transplants were excluded. Data extracted for analysis included number of trial centers (single vs. multi), use of an innovative dose-escalation design, use of expansion cohorts (i.e. phase Ib, phase II), and inclusion of patients over age 80. Innovative dose-escalation design was defined as designs that used a model based approach or a more sophisticated rule based design other than traditional 3+3 Phase I designs. Studies published from 2005-2010 were compared with studies from 2011-2015. The chi-squared test was used to compare the analyzed binary variables.
Results:
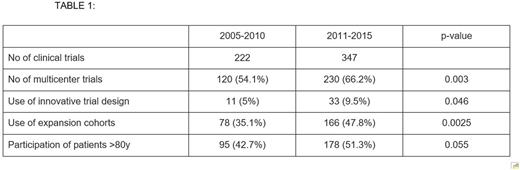
We identified 568 phase 1 clinical trials in malignant hematology meeting specified criteria which had published methodologies available for review. Results are summarized in Table 1. There were more phase I trials published in 2011-2015 (347) compared with 2005-2010 (222). From 2011-2015, compared to the 2005-2010 period, there was a significant increase in the proportion of trials which were multicenter (66.2% vs 54.1%, p=0.003), trials utilizing innovative dose escalation designs (9.5% vs 5%, p=0.046), and trials utilizing expansion cohorts (47.8% vs 35.1%, p=0.0025). There was a non-significant trend toward increased number of trials which included patients over age 80 (51.3% vs. 42.7%, p=0.055).
Conclusions:
Among phase I trials in malignant hematology over 2005-2015, there has been an increase in use of novel methodologies including multicenter collaboration, innovative dose escalation designs, and use of expansion cohorts, although many of these features remain underutilized.
The number of studies has increased at a rapid rate, likely reflecting recent development of molecularly targeted agents and immunotherapy. Although it is possible that the prevalence of many hematologic malignancies is increasing, it is unlikely that it matches the rate of growth of phase 1 trials, thus making it important to utilize more efficient trial designs in order to meet enrollment goals.
Yee: Novartis Canada: Honoraria; Astex: Research Funding; Celgene Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Oncoethix: Research Funding. Chavez: Janssen: Speakers Bureau; Abbvie: Speakers Bureau; Kite: Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal